In [1]:
from xv.chemistry.physical import ElementsManager
In [2]:
ke = ElementsManager()
ke
Out[2]:
2333468519296@ElementsManager
verbose = False
Details of elements
Minimum Grade: 6
Maximum Grade: 12
Examples
--------
ke = ElementsManager()
ke
ke.printProblemTypes()
ke.getRandomProblem()
ke.getRandomProblem(problem_type = 0)
...
ke.printProblem()
ke.printAnswer()
ke.printSolution()
doc_style: xv_doc
verbose = False
Details of elements
Minimum Grade: 6
Maximum Grade: 12
Examples
--------
ke = ElementsManager()
ke
ke.printProblemTypes()
ke.getRandomProblem()
ke.getRandomProblem(problem_type = 0)
...
ke.printProblem()
ke.printAnswer()
ke.printSolution()
doc_style: xv_doc
In [3]:
ke.printProblemTypes()
0. _problem_spd_orbitals 1. _problem_possible_quantum_numbers 2. _problem_electronic_config 3. _problem_stable_electronic_configs 4. _problem_electronic_config_noble_gases 5. _problem_valence_electronic_config 6. _problem_element_positive_ionization_energies_of_an_element 7. _problem_ionization_energy_analysis 8. _problem_electronic_config_based_props 9. _problem_element_isotopes 10. _problem_atomic_radii_analysis 11. _problem_bond_energy_inorganic_covalent_bonds 12. _problem_bond_energy_organic_covalent_bonds 13. _problem_element_ionic_radii 14. _problem_element_ionization_energies_of_elements 15. _problem_oxides_of_an_element 16. _problem_element_properties
In [4]:
from IPython.display import HTML
n = len(ke._problemTemplates)
max_loop = 1
for j in range(0, max_loop):
for i in range(n):
problem_type = i
display(HTML(f"<h2>problem_type: {problem_type}/{n-1} (loop {j}/{max_loop-1})</h2>"))
ke.getRandomProblem(problem_type = problem_type, verbose = True)
display(ke.printProblem())
display(HTML(f"<h6>Answer:</h6>"))
display(ke.printAnswer())
display(HTML(f"<h6>Solution:</h6>"))
display(ke.printSolution())
pass
problem_type: 0/16 (loop 0/0)
Problem Template: _problem_spd_orbitals
Draw the following orbital structures:
- s orbitals
- p orbitals
- d orbitals
- Shapes of the 4f orbitals in 3D
- shape and relative size of 1s, 2s and 2p orbitals
Answer:
-
orbitals-s
s orbitals -
orbitals-p
p orbitals -
orbitals-d
d orbitals -
orbitals-f
Shapes of the 4f orbitals in 3D -
a-level-orbitals-all
shape and relative size of 1s, 2s and 2p orbitals
Solution:
-
orbitals-s
s orbitals -
orbitals-p
p orbitals -
orbitals-d
d orbitals -
orbitals-f
Shapes of the 4f orbitals in 3D -
a-level-orbitals-all
shape and relative size of 1s, 2s and 2p orbitals
problem_type: 1/16 (loop 0/0)
Problem Template: _problem_possible_quantum_numbers
What are possible quantum numbers for the Principal quantum number 8 or R
Answer:
| Angular momentum | Magnetic moment | Spin | Electron filling order |
| 0 = s | 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 0 = 8 |
| 1 = p | -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 1 = 9 |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 1 = 9 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 1 = 9 | |
| 2 = d | -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 3 = f | -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 4 = g | -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 5 = h | -5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 |
| -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 6 = i | -6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 |
| -5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 7 = k | -7 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 |
| -6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 7 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 |
Solution:
Angular momentum quantum number
0, 1, 2, 3, ... n-1 where n is the principal quantum number
Magnetic moment quantum number
-m, -m+1, ... -1, 0, 1, ... m-1, m where m is the angular momentum quantum number
Spin quantum number
$\frac{1}{2}$, $- \frac{1}{2}$ for every magnetic moment quantum number
The electron filling
The electron filling order is sum of (Angular momentum quantum number) and (Magnetic moment quantum number).
If there is a tie, the electron will go to the lower angular momentum quantum number.
Quantum numbers for principal quantum number = 8 = R
| Angular momentum | Magnetic moment | Spin | Electron filling order |
| 0 = s | 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 0 = 8 |
| 1 = p | -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 1 = 9 |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 1 = 9 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 1 = 9 | |
| 2 = d | -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 2 = 10 | |
| 3 = f | -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 3 = 11 | |
| 4 = g | -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 4 = 12 | |
| 5 = h | -5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 |
| -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 5 = 13 | |
| 6 = i | -6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 |
| -5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 6 = 14 | |
| 7 = k | -7 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 |
| -6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| -1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 0 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 1 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 2 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 3 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 4 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 5 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 6 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 | |
| 7 | $\frac{1}{2}$, $- \frac{1}{2}$ | 8 + 7 = 15 |
problem_type: 2/16 (loop 0/0)
Problem Template: _problem_electronic_config
Write the following for the atom with atomic number 89:
1. Electronic configuration
2. Electronic configuration form with noble gas as core
3. Valence electrons
4. Detailed configuration
Answer:
Atomic symbol: Ac
Atomic number: 89
Name: Actinium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 6d1 7s2
2. Electronic configuration form with noble gas as core: [Rn] 6d1 7s2
3. Valence electrons: 7s2 6d1
4. Detailed configuration:
Atomic number: 89
Name: Actinium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 6d1 7s2
2. Electronic configuration form with noble gas as core: [Rn] 6d1 7s2
3. Valence electrons: 7s2 6d1
4. Detailed configuration:
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Solution:
Atomic symbol: Ac
Atomic number: 89
Name: Actinium
Note:It does not follow Madelung energy ordering rule, also called the n + l rule or aufbau approximation rule.
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 6d1 7s2
2. Electronic configuration form with noble gas as core: [Rn] 6d1 7s2
3. Valence electrons: 7s2 6d1
4. Detailed configuration:
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
problem_type: 3/16 (loop 0/0)
Problem Template: _problem_stable_electronic_configs
Electronic configuration of noble gases
Answer:
Electronic configuration of noble gases
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
Solution:
Electronic configuration of noble gases
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
problem_type: 4/16 (loop 0/0)
Problem Template: _problem_electronic_config_noble_gases
Electronic configuration of noble gases
Answer:
Electronic configuration of noble gases
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
Solution:
Electronic configuration of noble gases
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
He: 1s2
Ne: 1s2 2s2 2p6
Ar: 1s2 2s2 2p6 3s2 3p6
Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
Rn: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
problem_type: 5/16 (loop 0/0)
Problem Template: _problem_valence_electronic_config
Write the valence electrons configuration for atomic_number 24:
Answer:
Atomic symbol: Cr
Atomic number: 24
Name: Chromium
1. Valence electrons: 4s1 3d5
2. Valence electrons configuration:
3. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 3d5 4s1
4. Electronic configuration form with noble gas as core: [Ar] 3d5 4s1
Atomic number: 24
Name: Chromium
1. Valence electrons: 4s1 3d5
2. Valence electrons configuration:
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 3d5 4s1
4. Electronic configuration form with noble gas as core: [Ar] 3d5 4s1
Solution:
Atomic symbol: Cr
Atomic number: 24
Name: Chromium
1. Valence electrons: 4s1 3d5
2. Valence electrons configuration:
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 3d5 4s1
4. Electronic configuration form with noble gas as core: [Ar] 3d5 4s1
Note:It does not follow Madelung energy ordering rule, also called the n + l rule or aufbau approximation rule.
problem_type: 6/16 (loop 0/0)
Problem Template: _problem_element_positive_ionization_energies_of_an_element
Plot positive ionization energies of atomic number 39?
Answer:
Atomic symbol: Y
Atomic number: 39
Name: Yttrium
Electronic configuration form with noble gas as core: [Kr] 4d1 5s2
Atomic number: 39
Name: Yttrium
Electronic configuration form with noble gas as core: [Kr] 4d1 5s2
| 1+ | 2+ | 3+ | 4+ |
|---|---|---|---|
| 6.21726 | 12.224 | 20.52441 | 60.607 |
Solution:
Atomic symbol: Y
Atomic number: 39
Name: Yttrium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d1 5s2
2. Valence electrons: 5s2 4d1
3. Electronic configurations of its positive ions:
$Y^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d1 5s1
$Y^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d1
$Y^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
$Y^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
Electronic configuration form with noble gas as core: [Kr] 4d1 5s2
Atomic number: 39
Name: Yttrium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d1 5s2
2. Valence electrons: 5s2 4d1
3. Electronic configurations of its positive ions:
$Y^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d1 5s1
$Y^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d1
$Y^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
$Y^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
Electronic configuration form with noble gas as core: [Kr] 4d1 5s2
| 1+ | 2+ | 3+ | 4+ |
|---|---|---|---|
| 6.21726 | 12.224 | 20.52441 | 60.607 |
problem_type: 7/16 (loop 0/0)
Problem Template: _problem_ionization_energy_analysis
Plot ionization energies of atomic numbers 2 to 102.
Answer:
First Ionization Energies:
2: 24.587387936, 3: 5.391714761
3: 5.391714761, 4: 9.322699
4: 9.322699, 5: 8.298019
5: 8.298019, 6: 11.260296, 7: 14.53413
7: 14.53413, 8: 13.618054
8: 13.618054, 9: 17.42282, 10: 21.56454
10: 21.56454, 11: 5.1390767
11: 5.1390767, 12: 7.646235
12: 7.646235, 13: 5.9857684
13: 5.9857684, 14: 8.151683, 15: 10.486686
15: 10.486686, 16: 10.36001
16: 10.36001, 17: 12.96763, 18: 15.7596112
18: 15.7596112, 19: 4.34066354
19: 4.34066354, 20: 6.1131552, 21: 6.56149, 22: 6.82812
22: 6.82812, 23: 6.746187
23: 6.746187, 24: 6.76651, 25: 7.434018, 26: 7.9024678
26: 7.9024678, 27: 7.88101, 28: 7.639877
28: 7.639877, 29: 7.72638, 30: 9.394199
30: 9.394199, 31: 5.9993018
31: 5.9993018, 32: 7.899435, 33: 9.789
33: 9.789, 34: 9.752392
34: 9.752392, 35: 11.81381, 36: 13.9996049
36: 13.9996049, 37: 4.177128
37: 4.177128, 38: 5.6948672, 39: 6.21726, 40: 6.6339, 41: 6.75885, 42: 7.09243, 43: 7.119381, 44: 7.3605, 45: 7.4589, 46: 8.33686
46: 8.33686, 47: 7.576234
47: 7.576234, 48: 8.993822
48: 8.993822, 49: 5.7863552
49: 5.7863552, 50: 7.343917, 51: 8.608389, 52: 9.00966, 53: 10.45126, 54: 12.1298431
54: 12.1298431, 55: 3.893905548
55: 3.893905548, 56: 5.211664, 57: 5.5769
57: 5.5769, 58: 5.5386, 59: 5.473
59: 5.473, 60: 5.525, 61: 5.582, 62: 5.64371, 63: 5.670385, 64: 6.149796
64: 6.149796, 65: 5.8638
65: 5.8638, 66: 5.93905, 67: 6.0215, 68: 6.1077, 69: 6.18431, 70: 6.25416
70: 6.25416, 71: 5.425871
71: 5.425871, 72: 6.82507, 73: 7.54957, 74: 7.86403
74: 7.86403, 75: 7.83352
75: 7.83352, 76: 8.43823, 77: 8.96702
77: 8.96702, 78: 8.95883
78: 8.95883, 79: 9.225553, 80: 10.437504
80: 10.437504, 81: 6.108287
81: 6.108287, 82: 7.4166796
82: 7.4166796, 83: 7.285516
83: 7.285516, 84: 8.414, 85: 9.31751, 86: 10.7485
86: 10.7485, 87: 4.0727409
87: 4.0727409, 88: 5.278424, 89: 5.380226, 90: 6.3067
90: 6.3067, 91: 5.89
91: 5.89, 92: 6.19405, 93: 6.2655
93: 6.2655, 94: 6.0258, 95: 5.9738
95: 5.9738, 96: 5.9914, 97: 6.1978, 98: 6.2817, 99: 6.3676, 100: 6.5, 101: 6.58, 102: 6.65
Solution:
First Ionization Energies:
2: 24.587387936, 3: 5.391714761
3: 5.391714761, 4: 9.322699
4: 9.322699, 5: 8.298019
5: 8.298019, 6: 11.260296, 7: 14.53413
7: 14.53413, 8: 13.618054
8: 13.618054, 9: 17.42282, 10: 21.56454
10: 21.56454, 11: 5.1390767
11: 5.1390767, 12: 7.646235
12: 7.646235, 13: 5.9857684
13: 5.9857684, 14: 8.151683, 15: 10.486686
15: 10.486686, 16: 10.36001
16: 10.36001, 17: 12.96763, 18: 15.7596112
18: 15.7596112, 19: 4.34066354
19: 4.34066354, 20: 6.1131552, 21: 6.56149, 22: 6.82812
22: 6.82812, 23: 6.746187
23: 6.746187, 24: 6.76651, 25: 7.434018, 26: 7.9024678
26: 7.9024678, 27: 7.88101, 28: 7.639877
28: 7.639877, 29: 7.72638, 30: 9.394199
30: 9.394199, 31: 5.9993018
31: 5.9993018, 32: 7.899435, 33: 9.789
33: 9.789, 34: 9.752392
34: 9.752392, 35: 11.81381, 36: 13.9996049
36: 13.9996049, 37: 4.177128
37: 4.177128, 38: 5.6948672, 39: 6.21726, 40: 6.6339, 41: 6.75885, 42: 7.09243, 43: 7.119381, 44: 7.3605, 45: 7.4589, 46: 8.33686
46: 8.33686, 47: 7.576234
47: 7.576234, 48: 8.993822
48: 8.993822, 49: 5.7863552
49: 5.7863552, 50: 7.343917, 51: 8.608389, 52: 9.00966, 53: 10.45126, 54: 12.1298431
54: 12.1298431, 55: 3.893905548
55: 3.893905548, 56: 5.211664, 57: 5.5769
57: 5.5769, 58: 5.5386, 59: 5.473
59: 5.473, 60: 5.525, 61: 5.582, 62: 5.64371, 63: 5.670385, 64: 6.149796
64: 6.149796, 65: 5.8638
65: 5.8638, 66: 5.93905, 67: 6.0215, 68: 6.1077, 69: 6.18431, 70: 6.25416
70: 6.25416, 71: 5.425871
71: 5.425871, 72: 6.82507, 73: 7.54957, 74: 7.86403
74: 7.86403, 75: 7.83352
75: 7.83352, 76: 8.43823, 77: 8.96702
77: 8.96702, 78: 8.95883
78: 8.95883, 79: 9.225553, 80: 10.437504
80: 10.437504, 81: 6.108287
81: 6.108287, 82: 7.4166796
82: 7.4166796, 83: 7.285516
83: 7.285516, 84: 8.414, 85: 9.31751, 86: 10.7485
86: 10.7485, 87: 4.0727409
87: 4.0727409, 88: 5.278424, 89: 5.380226, 90: 6.3067
90: 6.3067, 91: 5.89
91: 5.89, 92: 6.19405, 93: 6.2655
93: 6.2655, 94: 6.0258, 95: 5.9738
95: 5.9738, 96: 5.9914, 97: 6.1978, 98: 6.2817, 99: 6.3676, 100: 6.5, 101: 6.58, 102: 6.65
problem_type: 8/16 (loop 0/0)
Problem Template: _problem_electronic_config_based_props
Write the following for the atom with atomic number 58:
1. Electronic configuration
2. Largest noble gas core
3. Electronic configuration form with noble gas as core
4. Valence electrons
5. Maximum number of shells
6. Last subshell
7. Electronic configurations of its positive ions
8. Electrons per shell
Answer:
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s2
2. Largest noble gas core: Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
3. Electronic configuration form with noble gas as core: [Xe] 4f1 5d1 6s2
4. Valence electrons: 6s2 4f1 5d1
5. Maximum number of shells: 6
6. Last subshell: $6s^{2}$
7. Electronic configurations of its positive ions:
$Ce^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s1
$Ce^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1
$Ce^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Ce^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Ce^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
8. Electrons per shell:
K: 2
L: 8
M: 18
N: 19
O: 9
P: 2
2. Largest noble gas core: Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
3. Electronic configuration form with noble gas as core: [Xe] 4f1 5d1 6s2
4. Valence electrons: 6s2 4f1 5d1
5. Maximum number of shells: 6
6. Last subshell: $6s^{2}$
7. Electronic configurations of its positive ions:
$Ce^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s1
$Ce^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1
$Ce^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Ce^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Ce^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
8. Electrons per shell:
K: 2
L: 8
M: 18
N: 19
O: 9
P: 2
Solution:
Atomic symbol: Ce
Atomic number: 58
Name: Cerium
Note:It does not follow Madelung energy ordering rule, also called the n + l rule or aufbau approximation rule.
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s2
2. Largest noble gas core: Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
3. Electronic configuration form with noble gas as core: [Xe] 4f1 5d1 6s2
4. Valence electrons: 6s2 4f1 5d1
5. Maximum number of shells: 6
6. Last subshell: $6s^{2}$
7. Electronic configurations of its positive ions:
$Ce^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s1
$Ce^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1
$Ce^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Ce^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Ce^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
8. Electrons per shell:
K: 2
L: 8
M: 18
N: 19
O: 9
P: 2
Atomic number: 58
Name: Cerium
Note:It does not follow Madelung energy ordering rule, also called the n + l rule or aufbau approximation rule.
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s2
2. Largest noble gas core: Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
3. Electronic configuration form with noble gas as core: [Xe] 4f1 5d1 6s2
4. Valence electrons: 6s2 4f1 5d1
5. Maximum number of shells: 6
6. Last subshell: $6s^{2}$
7. Electronic configurations of its positive ions:
$Ce^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s1
$Ce^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1
$Ce^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Ce^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Ce^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
8. Electrons per shell:
K: 2
L: 8
M: 18
N: 19
O: 9
P: 2
problem_type: 9/16 (loop 0/0)
Problem Template: _problem_element_isotopes
What are isotopes of atomic number 76?
Answer:
| mass_number | 184 | 186 | 187 | 188 | 189 | 190 | 192 |
|---|---|---|---|---|---|---|---|
| protons | 76 | 76 | 76 | 76 | 76 | 76 | 76 |
| neutrons | 108 | 110 | 111 | 112 | 113 | 114 | 116 |
| electrons | 76 | 76 | 76 | 76 | 76 | 76 | 76 |
Solution:
Isotopes have same atomic number but different mass numbers.
abundance: Relative Abundance
g_factor: Nuclear g-factor8
half_life: Half life of the isotope
half_life_unit: Unit in which the half life is given
is_radioactive: Is the isotope radioactive
mass: Atomic mass (Da)
mass_number: Mass number of the isotope
mass_uncertainty: Uncertainty of the atomic mass
spin: Nuclear spin quantum number
quadrupole_moment: Nuclear electric quadrupole moment8 (b [100 fm^2])
| mass_number | 184 | 186 | 187 | 188 | 189 | 190 | 192 |
|---|---|---|---|---|---|---|---|
| atomic_number | 76 | 76 | 76 | 76 | 76 | 76 | 76 |
| mass | 183.952489 | 185.95384 | 186.95575 | 187.95584 | 188.95814 | 189.95844 | 191.96148 |
| abundance | 0.0002 | 0.0159 | 0.0196 | 0.1324 | 0.1615 | 0.2626 | 0.4078 |
| mass_uncertainty | 0.000009 | 0.00001 | 0.00001 | 0.00001 | 0.00002 | 0.00002 | 0.00002 |
| is_radioactive | True | True | False | False | False | False | False |
| half_life | - | 2000000000000000.0 | - | - | - | - | - |
| half_life_unit | - | - | - | - | - | - | - |
| spin | 0.0 | 0.0 | 0.5 | 0.0 | 1.5 | 0.0 | 0.0 |
| g_factor | 0.0 | 0.0 | 0.129304 | 0.0 | 0.439956 | 0.0 | 0.0 |
| quadrupole_moment | 0.0 | 0.0 | 0.0 | 0.0 | 0.86 | 0.0 | 0.0 |
| protons | 76 | 76 | 76 | 76 | 76 | 76 | 76 |
| neutrons | 108 | 110 | 111 | 112 | 113 | 114 | 116 |
| electrons | 76 | 76 | 76 | 76 | 76 | 76 | 76 |
abundance: Relative Abundance
g_factor: Nuclear g-factor8
half_life: Half life of the isotope
half_life_unit: Unit in which the half life is given
is_radioactive: Is the isotope radioactive
mass: Atomic mass (Da)
mass_number: Mass number of the isotope
mass_uncertainty: Uncertainty of the atomic mass
spin: Nuclear spin quantum number
quadrupole_moment: Nuclear electric quadrupole moment8 (b [100 fm^2])
problem_type: 10/16 (loop 0/0)
Problem Template: _problem_atomic_radii_analysis
Plot and explain radii trend of atoms between atomic numbers 1 and 118
Answer:
Atomic Number
As atomic number increases, the positive charge of nucleus and
the negative charge of electrons increase.
Hence, the attraction force between them increases, causing radius to shrink.
A new shell
As a new electron shell is added to an atom, its size increases.
Type of radii available for plotting:
'empirical', 'Calculated', 'van der Waals', 'Covalent (single bond)', 'Covalent (triple bond)', 'Metallic'
See more..
Solution:
Atomic Number
As atomic number increases, the positive charge of nucleus and
the negative charge of electrons increase.
Hence, the attraction force between them increases, causing radius to shrink.
A new shell
As a new electron shell is added to an atom, its size increases.
Type of radii available for plotting:
'empirical', 'Calculated', 'van der Waals', 'Covalent (single bond)', 'Covalent (triple bond)', 'Metallic'
See more..
problem_type: 11/16 (loop 0/0)
_initProblemTemplate Error: 'BondEnergyHelper' object has no attribute 'use_cache'
Failed to get problemTemplate failed in 3 tries.
kwargs: {'problem_type': 11, 'verbose': True}
Traceback (most recent call last):
File "E:\Projects\pythonProjects\xv-km-lib\src\xv\km\kelements\_k_manager.py", line 111, in _initProblemTemplate
problemTemplate = getattr(self, problem_type_name)(*args, **kwargs)
File "E:\Projects\pythonProjects\xv-chemistry-lib\src\xv\chemistry\physical\_elements_manager.py", line 1004, in _problem_bond_energy_inorganic_covalent_bonds
svg_str = beh.plot_bond_energy_per_mol(df = selected_df)
File "E:\Projects\pythonProjects\xv-chemistry-lib\src\xv\chemistry\helper\_bond_energy_helper.py", line 195, in plot_bond_energy_per_mol
use_cache = self.use_cache
AttributeError: 'BondEnergyHelper' object has no attribute 'use_cache'
Failed to get random problem
Error: problem is not initialized.
None
Answer:
Error: problem is not initialized.
None
Solution:
Error: problem is not initialized.
None
problem_type: 12/16 (loop 0/0)
_initProblemTemplate Error: 'BondEnergyHelper' object has no attribute 'use_cache'
Failed to get problemTemplate failed in 3 tries.
kwargs: {'problem_type': 12, 'verbose': True}
Traceback (most recent call last):
File "E:\Projects\pythonProjects\xv-km-lib\src\xv\km\kelements\_k_manager.py", line 111, in _initProblemTemplate
problemTemplate = getattr(self, problem_type_name)(*args, **kwargs)
File "E:\Projects\pythonProjects\xv-chemistry-lib\src\xv\chemistry\physical\_elements_manager.py", line 1079, in _problem_bond_energy_organic_covalent_bonds
svg_str = beh.plot_bond_energy_per_mol()
File "E:\Projects\pythonProjects\xv-chemistry-lib\src\xv\chemistry\helper\_bond_energy_helper.py", line 195, in plot_bond_energy_per_mol
use_cache = self.use_cache
AttributeError: 'BondEnergyHelper' object has no attribute 'use_cache'
Failed to get random problem
Error: problem is not initialized.
None
Answer:
Error: problem is not initialized.
None
Solution:
Error: problem is not initialized.
None
problem_type: 13/16 (loop 0/0)
Problem Template: _problem_element_ionic_radii
What is crystal_radius of atomic number 16?
Answer:
Atomic symbol: S
Atomic number: 16
Name: Sulfur
Atomic number: 16
Name: Sulfur
| atomic_number | 16 | ||
|---|---|---|---|
| charge | -2 | 4 | 6 |
| coordination | |||
| I | NaN | NaN | NaN |
| II | NaN | NaN | NaN |
| III | NaN | NaN | NaN |
| IIIPY | NaN | NaN | NaN |
| IV | NaN | NaN | 26.0 |
| IVPY | NaN | NaN | NaN |
| IVSQ | NaN | NaN | NaN |
| IX | NaN | NaN | NaN |
| V | NaN | NaN | NaN |
| VI | 170.0 | 51.0 | 43.0 |
| VII | NaN | NaN | NaN |
| VIII | NaN | NaN | NaN |
| X | NaN | NaN | NaN |
| XI | NaN | NaN | NaN |
| XII | NaN | NaN | NaN |
| XIV | NaN | NaN | NaN |
Solution:
Atomic symbol: S
Atomic number: 16
Name: Sulfur
Atomic number: 16
Name: Sulfur
| atomic_number | 16 | ||
|---|---|---|---|
| charge | -2 | 4 | 6 |
| coordination | |||
| I | NaN | NaN | NaN |
| II | NaN | NaN | NaN |
| III | NaN | NaN | NaN |
| IIIPY | NaN | NaN | NaN |
| IV | NaN | NaN | 26.0 |
| IVPY | NaN | NaN | NaN |
| IVSQ | NaN | NaN | NaN |
| IX | NaN | NaN | NaN |
| V | NaN | NaN | NaN |
| VI | 170.0 | 51.0 | 43.0 |
| VII | NaN | NaN | NaN |
| VIII | NaN | NaN | NaN |
| X | NaN | NaN | NaN |
| XI | NaN | NaN | NaN |
| XII | NaN | NaN | NaN |
| XIV | NaN | NaN | NaN |
problem_type: 14/16 (loop 0/0)
Problem Template: _problem_element_ionization_energies_of_elements
Plot ionization energies of atomic numbers 58 to 68.
Answer:
| IE1 | IE2 | IE3 | IE4 | IE5 | IE6 | IE7 | IE8 | |
|---|---|---|---|---|---|---|---|---|
| atomic_number | ||||||||
| 58 | 5.538600 | 10.850 | 20.1974 | 36.906 | 65.55 | 77.6 | 91.0 | 106.0 |
| 59 | 5.473000 | 10.550 | 21.6237 | 38.980 | 57.50 | 82.0 | 97.0 | 112.0 |
| 60 | 5.525000 | 10.720 | 22.1400 | 40.400 | 60.00 | 84.0 | 99.0 | 114.0 |
| 61 | 5.582000 | 10.900 | 22.0000 | 41.000 | 61.69 | 85.0 | 101.0 | 116.0 |
| 62 | 5.643710 | 11.070 | 23.4000 | 41.400 | 62.66 | 90.0 | 103.0 | 118.0 |
| 63 | 5.670385 | 11.241 | 24.9000 | 42.700 | 63.00 | 88.0 | 105.0 | 120.0 |
| 64 | 6.149796 | 12.090 | 20.6300 | 44.000 | 64.76 | 89.0 | 106.0 | 123.0 |
| 65 | 5.863800 | 11.520 | 21.9100 | 39.360 | 66.50 | 90.0 | 108.0 | 125.0 |
| 66 | 5.939050 | 11.670 | 22.9300 | 41.400 | 62.08 | 93.0 | 110.0 | 127.0 |
| 67 | 6.021500 | 11.800 | 22.8400 | 42.500 | 63.93 | 95.0 | 112.0 | 129.0 |
| 68 | 6.107700 | 11.930 | 22.7400 | 42.700 | 65.10 | 96.0 | 114.0 | 131.0 |
Solution:
| IE1 | IE2 | IE3 | IE4 | IE5 | IE6 | IE7 | IE8 | |
|---|---|---|---|---|---|---|---|---|
| atomic_number | ||||||||
| 58 | 5.538600 | 10.850 | 20.1974 | 36.906 | 65.55 | 77.6 | 91.0 | 106.0 |
| 59 | 5.473000 | 10.550 | 21.6237 | 38.980 | 57.50 | 82.0 | 97.0 | 112.0 |
| 60 | 5.525000 | 10.720 | 22.1400 | 40.400 | 60.00 | 84.0 | 99.0 | 114.0 |
| 61 | 5.582000 | 10.900 | 22.0000 | 41.000 | 61.69 | 85.0 | 101.0 | 116.0 |
| 62 | 5.643710 | 11.070 | 23.4000 | 41.400 | 62.66 | 90.0 | 103.0 | 118.0 |
| 63 | 5.670385 | 11.241 | 24.9000 | 42.700 | 63.00 | 88.0 | 105.0 | 120.0 |
| 64 | 6.149796 | 12.090 | 20.6300 | 44.000 | 64.76 | 89.0 | 106.0 | 123.0 |
| 65 | 5.863800 | 11.520 | 21.9100 | 39.360 | 66.50 | 90.0 | 108.0 | 125.0 |
| 66 | 5.939050 | 11.670 | 22.9300 | 41.400 | 62.08 | 93.0 | 110.0 | 127.0 |
| 67 | 6.021500 | 11.800 | 22.8400 | 42.500 | 63.93 | 95.0 | 112.0 | 129.0 |
| 68 | 6.107700 | 11.930 | 22.7400 | 42.700 | 65.10 | 96.0 | 114.0 | 131.0 |
Atomic symbol: Ce
Atomic number: 58
Name: Cerium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s2
2. Valence electrons: 6s2 4f1 5d1
3. Electronic configurations of its positive ions:
$Ce^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1 6s1
$Ce^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1 5d1
$Ce^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Ce^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Ce^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 19
O: 9
P: 2
Atomic symbol: Pr
Atomic number: 59
Name: Praseodymium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3 6s2
2. Valence electrons: 6s2 4f3
3. Electronic configurations of its positive ions:
$Pr^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3 6s1
$Pr^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Pr^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Pr^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Pr^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Pr^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 21
O: 8
P: 2
Atomic symbol: Nd
Atomic number: 60
Name: Neodymium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4 6s2
2. Valence electrons: 6s2 4f4
3. Electronic configurations of its positive ions:
$Nd^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4 6s1
$Nd^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Nd^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Nd^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Nd^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Nd^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Nd^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 22
O: 8
P: 2
Atomic symbol: Pm
Atomic number: 61
Name: Promethium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5 6s2
2. Valence electrons: 6s2 4f5
3. Electronic configurations of its positive ions:
$Pm^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5 6s1
$Pm^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Pm^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Pm^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Pm^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Pm^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Pm^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Pm^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 23
O: 8
P: 2
Atomic symbol: Sm
Atomic number: 62
Name: Samarium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6 6s2
2. Valence electrons: 6s2 4f6
3. Electronic configurations of its positive ions:
$Sm^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6 6s1
$Sm^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6
$Sm^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Sm^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Sm^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Sm^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Sm^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Sm^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Sm^{9+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 24
O: 8
P: 2
Atomic symbol: Eu
Atomic number: 63
Name: Europium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7 6s2
2. Valence electrons: 6s2 4f7
3. Electronic configurations of its positive ions:
$Eu^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7 6s1
$Eu^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7
$Eu^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6
$Eu^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Eu^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Eu^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Eu^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Eu^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Eu^{9+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Eu^{10+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 25
O: 8
P: 2
Atomic symbol: Gd
Atomic number: 64
Name: Gadolinium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7 5d1 6s2
2. Valence electrons: 6s2 4f7 5d1
3. Electronic configurations of its positive ions:
$Gd^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7 5d1 6s1
$Gd^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7 5d1
$Gd^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7
$Gd^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6
$Gd^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Gd^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Gd^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Gd^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Gd^{9+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Gd^{10+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Gd^{11+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 25
O: 9
P: 2
Atomic symbol: Tb
Atomic number: 65
Name: Terbium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f9 6s2
2. Valence electrons: 6s2 4f9
3. Electronic configurations of its positive ions:
$Tb^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f9 6s1
$Tb^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f9
$Tb^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f8
$Tb^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7
$Tb^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6
$Tb^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Tb^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Tb^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Tb^{9+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Tb^{10+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Tb^{11+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Tb^{12+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 27
O: 8
P: 2
Atomic symbol: Dy
Atomic number: 66
Name: Dysprosium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f10 6s2
2. Valence electrons: 6s2 4f10
3. Electronic configurations of its positive ions:
$Dy^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f10 6s1
$Dy^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f10
$Dy^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f9
$Dy^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f8
$Dy^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7
$Dy^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6
$Dy^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Dy^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Dy^{9+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Dy^{10+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Dy^{11+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Dy^{12+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
$Dy^{13+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 28
O: 8
P: 2
Atomic symbol: Ho
Atomic number: 67
Name: Holmium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f11 6s2
2. Valence electrons: 6s2 4f11
3. Electronic configurations of its positive ions:
$Ho^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f11 6s1
$Ho^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f11
$Ho^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f10
$Ho^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f9
$Ho^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f8
$Ho^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7
$Ho^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6
$Ho^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Ho^{9+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Ho^{10+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Ho^{11+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Ho^{12+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
$Ho^{13+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 29
O: 8
P: 2
Atomic symbol: Er
Atomic number: 68
Name: Erbium
1. Electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f12 6s2
2. Valence electrons: 6s2 4f12
3. Electronic configurations of its positive ions:
$Er^{1+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f12 6s1
$Er^{2+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f12
$Er^{3+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f11
$Er^{4+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f10
$Er^{5+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f9
$Er^{6+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f8
$Er^{7+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f7
$Er^{8+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f6
$Er^{9+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f5
$Er^{10+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f4
$Er^{11+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f3
$Er^{12+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f2
$Er^{13+}$: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 4f1
4. Electrons per shell:
K: 2
L: 8
M: 18
N: 30
O: 8
P: 2
problem_type: 15/16 (loop 0/0)
Problem Template: _problem_oxides_of_an_element
Write oxides of element with atomic number 20
Answer:
oxides: $CaO$
Solution:
Atomic symbol: Ca
Atomic number: 20
Name: Calcium
electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2
oxidation states: 2
oxides: $CaO$
----------------------------------------------------------
Calcium oxide: $CaO$
molecular_weight = $56.0774$
monoisotopic_mass = $55.957504$
nominal_mass = $56$
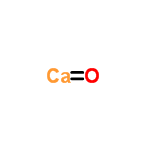
Calcium oxide: $CaO$
molecular_weight = $56.0774$
monoisotopic_mass = $55.957504$
nominal_mass = $56$
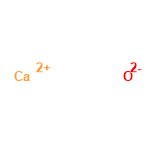
Atomic number: 20
Name: Calcium
electronic configuration: 1s2 2s2 2p6 3s2 3p6 4s2
oxidation states: 2
oxides: $CaO$
----------------------------------------------------------
Calcium oxide: $CaO$
molecular_weight = $56.0774$
monoisotopic_mass = $55.957504$
nominal_mass = $56$
Calcium oxide: $CaO$
molecular_weight = $56.0774$
monoisotopic_mass = $55.957504$
nominal_mass = $56$
problem_type: 16/16 (loop 0/0)
ec : <class 'mendeleev.econf.ElectronicConfiguration'> group : <class 'mendeleev.models.Group'> ionenergies : <class 'dict'> metadata : <class 'sqlalchemy.sql.schema.MetaData'> oxistates : <class 'list'> registry : <class 'sqlalchemy.orm.decl_api.registry'> sconst : <class 'dict'> Problem Template: _problem_element_properties
List the properties of the element with atomic number 34
Answer:
34 Se Selenium
Abundance in the Earth’s crust: 0.05 mg/kg
Abundance in the seas: 0.0002 mg/L
Annotations regarding the data:
Atomic number: 34
Atomic radius: 115.0 pm
Atomic radius by Rahm et al.: 224.00000000000003 pm
Atomic volume: 16.5 cm3/mol
Atomic weight1: 78.971
Atomic weight uncertainty1: 0.008
Block in periodic table: p
Boiling temperature: 958.1 K
C_6 dispersion coefficient in a.u.: 210.0 a.u.
C_6 dispersion coefficient in a.u. (Gould & Bučko): 233.0 a.u.
Chemical Abstracts Serice identifier: 7782-49-2
covalent_radius: 115.99999999999999
Covalent radius by Bragg: 117.0 pm
Covalent radius by Cerdero et al.2: 120.0 pm
Single bond covalent radius by Pyykko et al.: 115.99999999999999 pm
Double bond covalent radius by Pyykko et al.: 107.0 pm
Triple bond covalent radius by Pyykko et al.: 107.0 pm
Element color in CPK convention: #ff1493 HEX
Density at 295K: 4.809 g/cm3
Short description of the element: Metalloid element, belongs to group 16 of the periodic table. Multiple allotropic forms exist. Chemically resembles sulphur. Discovered in 1817 by Jons J. Berzelius.
Dipole polarizability: 28.9 a.u.
Dipole polarizability uncertainty: 1.0 a.u.
The discoverers of the element: Jöns Berzelius
The location where the element was discovered: Sweden
discovery_year: 1818
Ground state electron configuration: [Ar] 3d10 4s2 4p4
Electron affinity3: 2.02067 eV
electronegativity: 2.55
electronegativity_allen: 14.34
electronegativity_allred_rochow: 0.000516498216409037
electronegativity_cottrell_sutton: 0.24477294193486396
electronegativity_ghosh: 0.2240328
electronegativity_gordy: 0.05991379310344828
electronegativity_li_xue: {}
electronegativity_martynov_batsanov: 6.528391736612216
electronegativity_mulliken: 4.876196
electronegativity_nagle: 0.5921310056235959
electronegativity_pauling: 2.55
electronegativity_sanderson: 0.9659120763307081
electronegativity_scales: ['allen', 'allred-rochow', 'cottrell-sutton', 'ghosh', 'gordy', 'li-xue', 'martynov-batsanov', 'mulliken', 'nagle', 'pauling', 'sanderson']
Number of electrons: 34
Electrophilicity index: 2.2408492709619536 eV
Allen’s scale of electronegativity4: 14.34 eV
Ghosh’s scale of electronegativity: 0.2240328
Pauling’s scale of electronegativity: 2.55
Evaporation heat: 59.7 kJ/mol
Fusion heat: 5.23 kJ/mol
Geochemical classification: semi-volatile
Glawe’s number (scale): 95
Goldschmidt classification: chalcophile
group_id: 16
hardness: 3.865861
Heat of formation: 227.2 kJ/mol
init_on_load: None
Ionic and crystal radii in pm9: [IonicRadius(
atomic_number=34,
charge=-2,
coordination='VI',
crystal_radius=184.0,
econf='4p6',
id=375,
ionic_radius=198.0,
most_reliable=False,
origin="Pauling's (1960) crystal radius, ",
spin='',
), IonicRadius(
atomic_number=34,
charge=4,
coordination='VI',
crystal_radius=64.0,
econf='4s2',
id=376,
ionic_radius=50.0,
most_reliable=False,
origin='Ahrens (1952) ionic radius, ',
spin='',
), IonicRadius(
atomic_number=34,
charge=6,
coordination='IV',
crystal_radius=42.0,
econf='3d10',
id=377,
ionic_radius=28.000000000000004,
most_reliable=True,
origin='',
spin='',
), IonicRadius(
atomic_number=34,
charge=6,
coordination='VI',
crystal_radius=56.00000000000001,
econf='3d10',
id=378,
ionic_radius=42.0,
most_reliable=False,
origin='calculated, ',
spin='',
)] pm
Is the element radioactive: False
Isotopes: [
Element color in Jmol convention: #ffa100 HEX
Lattice constant: 4.36 Angstrom
Lattice structure code: HEX
mass: 78.971
Mass number: 80
mass_str: 78.971
Melting temperature: 490.0 K
Mendeleev’s number5: 101
Single-bond metallic radius: 117.0 pm
Metallic radius with 12 nearest neighbors: 140.0 pm
molar_heat_capacity: 25.363
Element color in MOCAS GV convention: #ffa100 HEX
Name in English: Selenium
Origin of the name: Greek: selênê (moon).
Number of neutrons (most abundant isotope): 46
nvalence: 6
oxides: ['SeO3', 'SeO2']
Period in periodic table: 4
Pettifor scale: 93
Number of protons: 34
screening_constants: [
Index to chemical series: Nonmetals
softness: 0.12933729381371964
Sources of the element: Obtained from lead, copper and nickel refining. Conducts electricity when struck by light.
Specific heat @ 20 C: 0.321 J/(g mol)
specific_heat_capacity: 0.321
Chemical symbol: Se
Thermal conductivity @25 C: 0.52 W/(m K)
Applications of the element: Light causes it to conduct electricity more easily. It is used in photoelectric cells, TV cameras, xerography machines and as a semiconductor in solar batteries and rectifiers. Also colors glass red.
Van der Waals radius: 190.0 pm
Van der Waals radius according to Alvarez7: 182.0 pm
Van der Waals radius according to Batsanov: 190.0 pm
Van der Waals radius according to Bondi: 190.0 pm
Van der Waals radius from the DREIDING FF: 403.0 pm
Van der Waals radius from the MM3 FF: 229.0 pm
Van der Waals radius from the UFF: 420.5 pm
zeff: 6.949999999999999
Solution:
34 Se Selenium
Abundance in the Earth’s crust: 0.05 mg/kg
Abundance in the seas: 0.0002 mg/L
Annotations regarding the data:
Atomic number: 34
Atomic radius: 115.0 pm
Atomic radius by Rahm et al.: 224.00000000000003 pm
Atomic volume: 16.5 cm3/mol
Atomic weight1: 78.971
Atomic weight uncertainty1: 0.008
Block in periodic table: p
Boiling temperature: 958.1 K
C_6 dispersion coefficient in a.u.: 210.0 a.u.
C_6 dispersion coefficient in a.u. (Gould & Bučko): 233.0 a.u.
Chemical Abstracts Serice identifier: 7782-49-2
covalent_radius: 115.99999999999999
Covalent radius by Bragg: 117.0 pm
Covalent radius by Cerdero et al.2: 120.0 pm
Single bond covalent radius by Pyykko et al.: 115.99999999999999 pm
Double bond covalent radius by Pyykko et al.: 107.0 pm
Triple bond covalent radius by Pyykko et al.: 107.0 pm
Element color in CPK convention: #ff1493 HEX
Density at 295K: 4.809 g/cm3
Short description of the element: Metalloid element, belongs to group 16 of the periodic table. Multiple allotropic forms exist. Chemically resembles sulphur. Discovered in 1817 by Jons J. Berzelius.
Dipole polarizability: 28.9 a.u.
Dipole polarizability uncertainty: 1.0 a.u.
The discoverers of the element: Jöns Berzelius
The location where the element was discovered: Sweden
discovery_year: 1818
Ground state electron configuration: [Ar] 3d10 4s2 4p4
Electron affinity3: 2.02067 eV
electronegativity: 2.55
electronegativity_allen: 14.34
electronegativity_allred_rochow: 0.000516498216409037
electronegativity_cottrell_sutton: 0.24477294193486396
electronegativity_ghosh: 0.2240328
electronegativity_gordy: 0.05991379310344828
electronegativity_li_xue: {}
electronegativity_martynov_batsanov: 6.528391736612216
electronegativity_mulliken: 4.876196
electronegativity_nagle: 0.5921310056235959
electronegativity_pauling: 2.55
electronegativity_sanderson: 0.9659120763307081
electronegativity_scales: ['allen', 'allred-rochow', 'cottrell-sutton', 'ghosh', 'gordy', 'li-xue', 'martynov-batsanov', 'mulliken', 'nagle', 'pauling', 'sanderson']
Number of electrons: 34
Electrophilicity index: 2.2408492709619536 eV
Allen’s scale of electronegativity4: 14.34 eV
Ghosh’s scale of electronegativity: 0.2240328
Pauling’s scale of electronegativity: 2.55
Evaporation heat: 59.7 kJ/mol
Fusion heat: 5.23 kJ/mol
Geochemical classification: semi-volatile
Glawe’s number (scale): 95
Goldschmidt classification: chalcophile
group_id: 16
hardness: 3.865861
Heat of formation: 227.2 kJ/mol
init_on_load: None
Ionic and crystal radii in pm9: [IonicRadius(
atomic_number=34,
charge=-2,
coordination='VI',
crystal_radius=184.0,
econf='4p6',
id=375,
ionic_radius=198.0,
most_reliable=False,
origin="Pauling's (1960) crystal radius, ",
spin='',
), IonicRadius(
atomic_number=34,
charge=4,
coordination='VI',
crystal_radius=64.0,
econf='4s2',
id=376,
ionic_radius=50.0,
most_reliable=False,
origin='Ahrens (1952) ionic radius, ',
spin='',
), IonicRadius(
atomic_number=34,
charge=6,
coordination='IV',
crystal_radius=42.0,
econf='3d10',
id=377,
ionic_radius=28.000000000000004,
most_reliable=True,
origin='',
spin='',
), IonicRadius(
atomic_number=34,
charge=6,
coordination='VI',
crystal_radius=56.00000000000001,
econf='3d10',
id=378,
ionic_radius=42.0,
most_reliable=False,
origin='calculated, ',
spin='',
)] pm
Is the element radioactive: False
Isotopes: [
Element color in Jmol convention: #ffa100 HEX
Lattice constant: 4.36 Angstrom
Lattice structure code: HEX
mass: 78.971
Mass number: 80
mass_str: 78.971
Melting temperature: 490.0 K
Mendeleev’s number5: 101
Single-bond metallic radius: 117.0 pm
Metallic radius with 12 nearest neighbors: 140.0 pm
molar_heat_capacity: 25.363
Element color in MOCAS GV convention: #ffa100 HEX
Name in English: Selenium
Origin of the name: Greek: selênê (moon).
Number of neutrons (most abundant isotope): 46
nvalence: 6
oxides: ['SeO3', 'SeO2']
Period in periodic table: 4
Pettifor scale: 93
Number of protons: 34
screening_constants: [
Index to chemical series: Nonmetals
softness: 0.12933729381371964
Sources of the element: Obtained from lead, copper and nickel refining. Conducts electricity when struck by light.
Specific heat @ 20 C: 0.321 J/(g mol)
specific_heat_capacity: 0.321
Chemical symbol: Se
Thermal conductivity @25 C: 0.52 W/(m K)
Applications of the element: Light causes it to conduct electricity more easily. It is used in photoelectric cells, TV cameras, xerography machines and as a semiconductor in solar batteries and rectifiers. Also colors glass red.
Van der Waals radius: 190.0 pm
Van der Waals radius according to Alvarez7: 182.0 pm
Van der Waals radius according to Batsanov: 190.0 pm
Van der Waals radius according to Bondi: 190.0 pm
Van der Waals radius from the DREIDING FF: 403.0 pm
Van der Waals radius from the MM3 FF: 229.0 pm
Van der Waals radius from the UFF: 420.5 pm
zeff: 6.949999999999999
<Figure size 1152x432 with 0 Axes>
In [ ]:
In [ ]:
In [ ]: